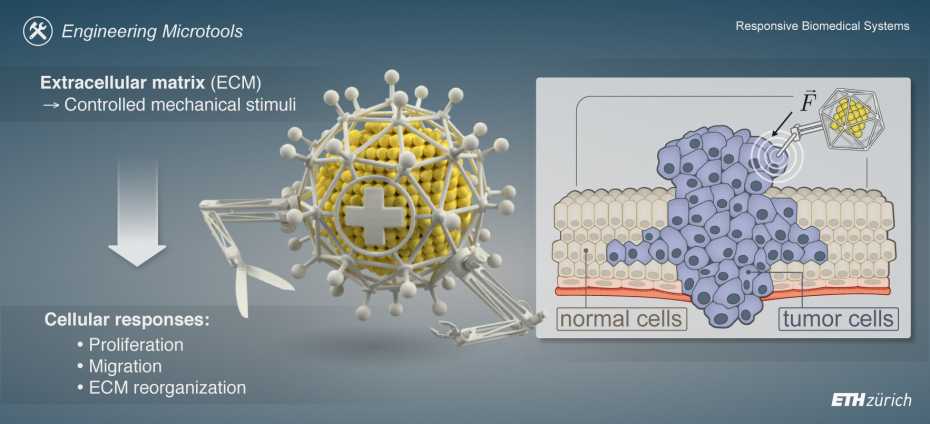
Engineering Microrobots to Probe Cells and Tissues
The extracellular matrix (ECM) is an essential component of healthy tissue that provides structure and support. However, in maladies including cancer, it is now understood that the ECM can also play a harmful role in disease progression and that characteristics of the tumor micro-environment arise in part from mechanical interactions between malignant and non-transformed cells within the ECM.
Understanding the factors that contribute to tumor initiation, growth, and migration is crucial for developing effective diagnostic and therapeutic tools. While it is known that the ECM changes in tumors, resulting in a stiffer tissue, the lack of tools to precisely control the mechanical stimuli at the cellular level limits the study of how different mechanical cues impact tumor cell behavior and their potential implications for therapeutic strategies.
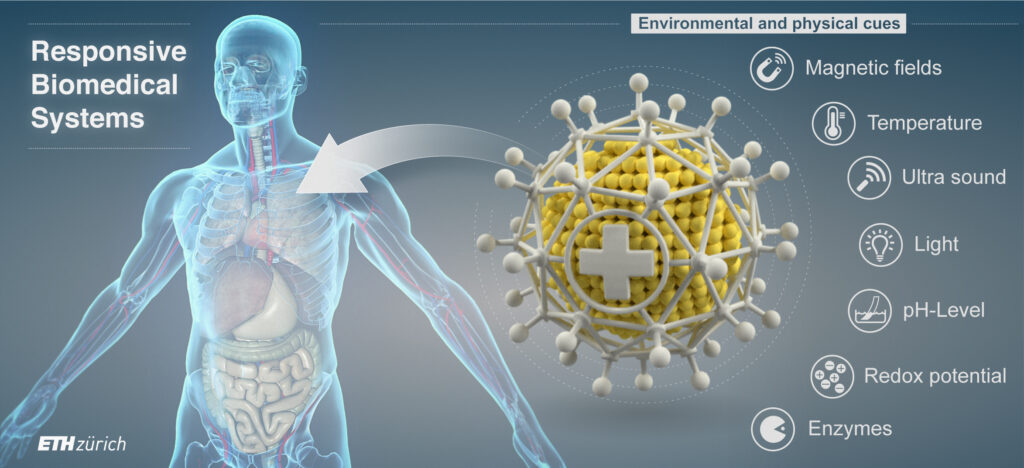
Our research in this field seeks to uncover new targets for treatment and mechanical cues that can be integrated into the design of diagnostic and therapeutic nanosystems. By using models that mimic tumors or simply the ECM, we are incorporating magnetic microrobots to apply controlled mechanical stimuli via custom magnetic instrumentation. This allows us to infer local mechanical properties, study cellular responses, such as proliferation, migration, and ECM reorganization, to better understand the influence of mechanical cues on tumor cell behavior.
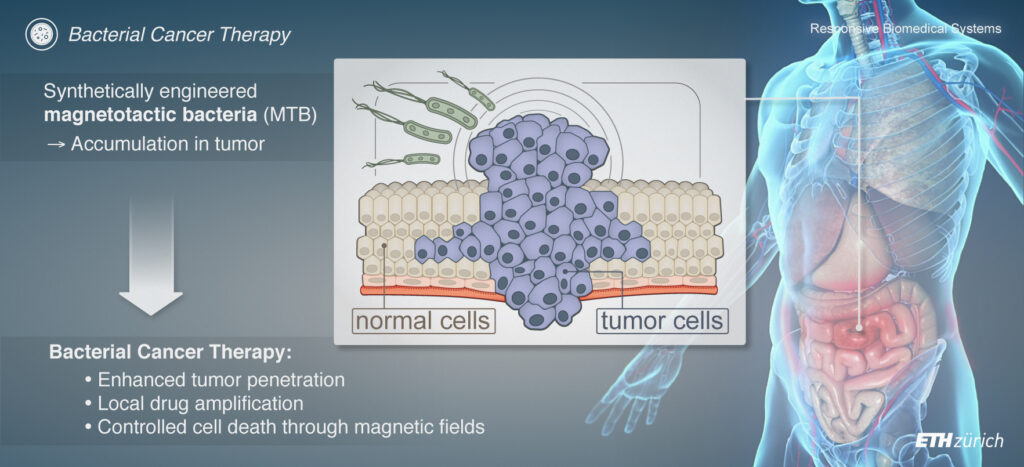
Biohybrid Microrobots for Drug Delivery
Targeted cancer therapy has brought new clinical approaches to the forefront, including the use of antibodies, small molecules, anti-angiogenics, and antivirals. However, these strategies are limited by the challenges of tumor accumulation and penetration.
Recently, synthetic biology has revitalized the idea of using living organisms to fight cancer. For instance, some bacteria can naturally accumulate in tumors, and can be engineered to induce local cytotoxicity while remaining harmless to the host. Clinical trials have demonstrated the safety and tolerance of strains such as E. coli and S. typhimurium for cancer therapy. S. typhimurium was initially shown to combat tumors through the recruitment of the host immune system and competition with cancer cells for nutrients, and later through the engineered production of therapeutic cargo through simple genetic modifications.
Synthetic biology is now taking this approach a step further by using genetic circuits with sophisticated sensing and delivery capabilities to enable bacteria to self-regulate therapeutic cargo production in response to tumor-specific stimuli.
Despite these exciting advances, there are still challenges in engineering bacteria for tumor therapy, such as safety, robust colonisation via effective delivery of tolerable doses as well as improving penetration of tumors. To address these hurdles, we are working on developing controllable, living biohybrid microrobots based on bacteria. This involves using either magnetotactic bacteria, which naturally produce magnetic particles, or functionalizing non-magnetic bacteria with magnetic nanomaterials for wireless magnetic control. These biohybrid microrobots are equipped with drug-filled nanoliposomes and/or therapeutic genetic circuits and are controlled and tracked using custom-built magnetic instrumentation and algorithms. This approach represents a promising, controllable, and biologically-based therapeutic platform for cancer treatment.
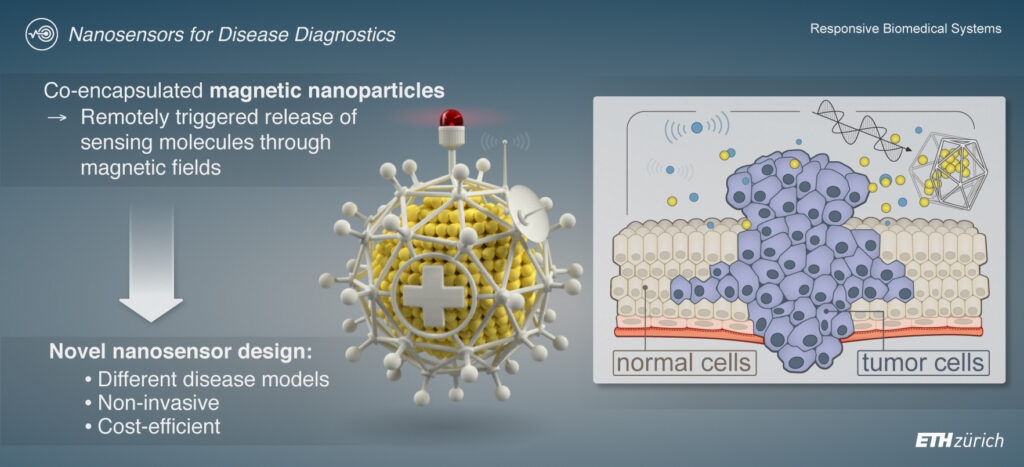
Micro-and Nanorobots for Disease Diagnostics
The field of personalized medicine is constantly evolving, and new diagnostic tools are needed to understand an individual patient’s disease state, inform clinical decision-making, and monitor therapeutic response. In previous work at MIT, we developed a nanosensor that can identify tumor-specific enzymatic activity profiles, such as from proteases, a crucial class of enzymes, through localized sampling of the tumor microenvironment.
Our nanosensors are composed of thermosensitive liposomes containing functionalized biomarker substrates that are selectively unveiled at the target site by the heat dissipation of co-encapsulated magnetic nanoparticles (MNPs) in response to the application of an alternating magnetic field. A detection assay is then used to quantify the amount of cleaved marker substrates excreted in the urine, a reading that is directly correlated with specific protease activity in the tumor. The spatiotemporally controlled system has been used to measure in vivo tumor protease activity, revealing differences in enzymatic profiles between two mouse models of human colorectal cancer.
At RBSL, we continue to explore the potential of these and other concepts to develop micro- and nanosensors and mechanisms to control them and communicate diagnostic signals in a non-invasive and cost-efficient manner. For instance, we are designing protease-responsive ultrasound contrast agents (PRUCAs) that can report protease activity through a change in the nonlinear backscattering signal of incoming acoustic waves. Our goal is to assess local protease activity by administering PRUCAs to patients and applying an ultrasound probe to a body area of interest. Additionally, we are developing nanosensors that detect protease activity, coupled with magnetic detection mechanisms, for in vivo use or as convenient benchtop readout systems.